-
Caribou Biosciences Presents Encouraging Clinical Data from CB-010 ANTLER Phase 1 Trial in Second-line LBCL Patients at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting
来源: Nasdaq GlobeNewswire / 02 6月 2024 19:00:00 America/New_York
-- CB-010 allogeneic CAR-T cell therapy with partial HLA matching has potential to rival efficacy and safety profile of approved autologous CAR-T cell therapies --
-- 14.4 months median PFS in ANTLER patients with partial HLA matching (≥4 alleles) --
-- Plan to enroll ~20 additional 2L LBCL patients in ANTLER to confirm that partial HLA matching improves patient outcomes; initial data expected in H1 2025 --
-- Caribou expects to initiate a pivotal trial for CB-010 in H2 2025, upon confirmation of improved outcomes in partially HLA matched cohort --
-- Off-the-shelf CB-010 is partially HLA matched to patient within current screening timelines --
-- KOL webcast discussion of data from 46 ANTLER patients scheduled for today at 7:00 pm CDT --
BERKELEY, Calif., June 02, 2024 (GLOBE NEWSWIRE) -- Caribou Biosciences, Inc. (Nasdaq: CRBU), a leading clinical-stage CRISPR genome-editing biopharmaceutical company, today presented updated clinical data from the ongoing ANTLER Phase 1 trial that indicates a single dose of CB-010, a readily available, off-the-shelf anti-CD19 CAR-T cell therapy with a PD-1 knockout, has the potential to rival the safety, efficacy, and durability of approved autologous CAR-T cell therapies. The clinical results are being presented during a poster presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting.
“The Phase 1 data from the ANTLER trial continues to be encouraging in terms of both safety and efficacy of an allogeneic CAR-T cell therapy,” said Boyu Hu, MD, director of lymphoma and CLL in the division of hematology and hematologic malignancies at the University of Utah and an investigator on the ANTLER trial. “The partial human leukocyte antigen, or HLA, matching strategy is incredibly intriguing and further evaluation is supported by the ASCO data presentation. As many patients in ANTLER were enrolled due to rapid disease progression that prohibited waiting for an autologous CAR-T cell therapy, I look forward to enrolling patients who will receive partially HLA matched CB-010 in this ongoing trial.”
In ANTLER, three dose levels of CB-010 were evaluated (40x106, 80x106, and 120x106 CAR-T cells) in a total of 46 patients. In dose escalation, 16 patients with multiple subtypes of aggressive relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL) were enrolled, and in dose expansion, 30 patients with second-line large B cell lymphoma (2L LBCL) were enrolled. As of an April 1, 2024 data cutoff date, results demonstrated:
- CB-010 was generally well tolerated. No Grade 3 or higher cytokine release syndrome (CRS) and no graft-versus-host disease (GvHD) was observed.
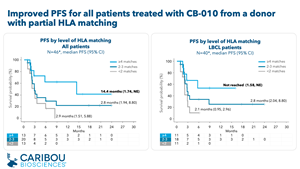
- A retrospective analysis of all patient data demonstrated that patients who received a dose of CB-010 manufactured from a donor with ≥4 matching HLA alleles (referred to as partial HLA matching) showed improved progression free survival (PFS). Results from patients who received partially HLA matched CB-010 include:
- Median PFS of 14.4 months (95% CI: 1.74, not estimable [NE]) was observed in patients treated with CB-010 with ≥4 HLA matches (N=13), compared to 2.8 months (95% CI: 2.10, 3.48) for patients treated with CB-010 with ≤3 HLA matches (N=33).
- In patients with LBCL who received CB-010 with ≥4 HLA matches (N=11, including N=10 2L LBCL and N=1 3L LBCL), median PFS has not been reached (95% CI: 1.58, NE).
- Translational data on CB-010:
- Pharmacokinetic (PK) data showed that higher numbers of matched HLA alleles between the CB-010 donor and recipient patient correlated with increased CAR-T cell expansion and persistence compared to lower numbers of matched HLA alleles.
- Pharmacodynamic (PD) data showed that a single dose of CB-010 resulted in extended B cell aplasia (~114 days) and a rapid recovery of the patient’s endogenous T and NK cells (~3 weeks).
- Based on the overall safety, efficacy, and translational data analyzed, 80x106 CAR-T cells was selected as the recommended Phase 2 dose (RP2D) for CB-010.
“We are excited to see that patients who receive partially HLA matched CB-010 have improved efficacy and durability outcomes that are on par with approved autologous CAR-T cell therapies,” said Rachel Haurwitz, PhD, Caribou’s president and chief executive officer. “We next plan to prospectively evaluate this compelling observation by enrolling approximately 20 additional 2L LBCL patients, in either the inpatient or outpatient treatment setting, and we will ensure that they receive a partially matched (≥4 HLA matches) dose of CB-010. We are also excited to open the ANTLER study for the first time to patients who have relapsed following any prior CD19-targeted therapy in a proof-of-concept cohort for up to 10 patients. We expect to report initial data from both the 2L LBCL and CD19 relapsed cohorts in the first half of 2025 and, upon confirmation of improved outcomes in additional patients receiving a partially HLA matched dose of CB-010, we plan to initiate a pivotal Phase 3 clinical trial in 2L LBCL patients, including patients regardless of HLA type, in the second half of 2025.”
ANTLER Phase 1 trial of CB-010 – median PFS analyses
A photo accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/893722aa-a457-4e0f-a27e-457bf8b2c0d3
CI: confidence interval; HLA: human leukocyte antigen; NE: not estimable; partial HLA matching: patient has ≥4 HLA alleles that match donor T cells used for CB-010 manufacturing
* Retrospective analysis of HLA allele matching for class I and class II antigens
ANTLER Phase 1 clinical trial as of April 1, 2024 cutoff date, data collection ongoingANTLER Phase 1 trial of CB-010 – response data
Endpoints
(N, %)All patients
≤3 HLA matches
(N=33)All patients
≥4 HLA matches
(N=13)LBCL
≥4 HLA matches
(N=11)Overall response rate (ORR) 23 (69%) 12 (92%) 10 (91%) Duration of response (DoR), median months (range) 2.0 (1-23+) 13.5 (1-23+) NR (1-15+) Complete response (CR) rate 15 (45%) 6 (46%) 4 (36%) Duration of CR, median months (range) 5.0 (1-23+) NR (5-23+) NR (5-15+) 6-month PFS 25% 62% 53% PFS, median months (range) 2.8 (1-24+) 14.4 (2-24+) NR (2-16+) HLA: human leukocyte antigen; NR: not reached; PFS: progression free survival
ANTLER Phase 1 clinical trial as of April 1, 2024 cutoff date, data collection ongoingANTLER Phase 1 trial of CB-010 – safety data
All treated
(N=46)Any grade
(n, %)Grade ≥3
(n, %)Prolonged cytopenias 9 (20)1 9 (20)1 CRS 26 (57)2 0 (0) Infections 22 (47)3 10 (22)3 ICANS 10 (22)4 3 (7)5 Hemophagocytic lymphohistiocytosis (HLH) 1 (2) 0 GvHD 0 0 CRS: cytokine release syndrome; GvHD: graft-versus-host disease; ICANS: immune effector cell-associated neurotoxicity syndrome
There were five patient deaths due to adverse events following CB-010 infusion; 4 were unrelated to CB-010 treatment and 1 death possibly related to CB-010 per investigator due to complications of a bladder perforation in the context of BK virus hemorrhagic cystitis
1 Prolonged cytopenias are defined as grade 3 or higher events lasting beyond 30 days following CB-010 infusion; 37/46 (80%) of patients recovered from cytopenias to grade ≤2 by day 35 post CB-010 treatment
2 Median time of onset was 3 days (range 0-22), and median duration was 3 days (range 1-19)
3 Infection events reported were on or after CB-010 infusion, with highest grade reported per patient; median onset 8 days (range 0-279) and median duration is 14 days (range 1-239)
4 Median time of onset was 7.5 days (range 6-34), and median duration was 2 days (range 1-27)
5 2 Grade 3 and 1 Grade 4; all resolved with supportive care. Median time of onset was 8 days and median duration was 2 days
ANTLER Phase 1 clinical trial as of April 1, 2024 cutoff date, data collection ongoingBased on these encouraging data, Caribou plans to enroll approximately 20 additional 2L LBCL patients in ANTLER to prospectively confirm that partial HLA matching improves patient outcomes. The patient HLA allele typing occurs within the current screening timelines.
“Integrating the partial HLA matching into manufacturing for CB-010 is straightforward, enabling Caribou to deliver CB-010 as a readily available off-the-shelf CAR-T cell therapy that can serve a broad patient population,” said Tim Kelly, Caribou’s chief technology officer. “In our planned 2L LBCL pivotal Phase 3 trial, we will provide the best possible matched dose of CB-010 to each patient based on lot availability. With at least 13 manufacturing batches of CB-010 on hand, we expect that approximately 90% of all patients who could enroll in our trial would receive a dose of CB-010 with ≥4 matched alleles.”
Webcast conference call Sunday, June 2, at 7:00 pm CDT
Caribou will host a live webcast on Sunday, June 2, at 7:00 pm CDT for a discussion with KOLs and management on the CB-010 ANTLER Phase 1 data presentation. The presenters will include:- Boyu Hu, MD, director of lymphoma and CLL in the division of hematology and hematologic malignancies, University of Utah
- Mehdi Hamadani, MD, professor of medicine, section chief of hematologic malignancies, Medical College of Wisconsin
- Rachel Haurwitz, PhD, president and chief executive officer, Caribou Biosciences
Additional participants from Caribou Biosciences include:
- Steve Kanner, PhD, chief scientific officer
- Jason O’Byrne, chief financial officer
- Kike Zudaire, PhD, senior vice president, translational sciences and therapeutic discovery
- Tonia Nesheiwat, PharmD, vice president of medical affairs and project leadership
The listen-only webcast with an accompanying presentation will be accessible under Events in the Investors section of Caribou’s website. The archived audio webcast will be available on the company’s website following the call and will be available for 30 days.
ASCO poster presentation on Monday, June 3, 9:00 am-12:00 pm CDT
Details of the ANTLER poster presentation at the 2024 ASCO Annual Meeting are as follows:Title: A CRISPR-edited allogeneic anti-CD19 CAR-T cell therapy with a PD-1 knockout (CB-010) in patients with relapsed/refractory B cell non-Hodgkin lymphoma (r/r B-NHL): Updated Phase 1 results from the ANTLER trial
Presenter: Boyu Hu, MD, assistant professor, director of lymphoma and CLL, division of hematology and hematologic malignancies, Huntsman Cancer Institute at the University of Utah
Date and time: Monday, June 3, 2024, 9:00 am-12:00 pm CDT
Session: Hematologic Malignancies – Lymphoma and CLL
Location: Hall A, Poster Board 8, McCormick Place, Chicago
Abstract number: 7025About CB-010
CB-010 is the lead clinical-stage product candidate from Caribou’s allogeneic CAR-T cell therapy platform, and it is being evaluated in patients with relapsed or refractory B cell non-Hodgkin lymphoma (r/r B-NHL) in the ongoing ANTLER Phase 1 clinical trial and will be evaluated in patients with lupus nephritis (LN) and extrarenal lupus (ERL) in the GALLOP Phase 1 clinical trial. In ANTLER, Caribou is enrolling second-line patients with large B cell lymphoma (LBCL) comprised of different subtypes of aggressive r/r B-NHL (DLBCL NOS, PMBCL, HGBL, tFL, and tMZL). To Caribou’s knowledge, CB-010 is the first allogeneic CAR-T cell therapy in the clinic with a PD-1 knockout, a genome-editing strategy designed to improve activity against diseases by limiting premature CAR-T cell exhaustion. CB-010 is also, to Caribou’s knowledge, the first anti-CD19 allogeneic CAR-T cell therapy to be evaluated in the second-line LBCL setting and, for r/r B-NHL, CB-010 has been granted Regenerative Medicine Advanced Therapy (RMAT), Fast Track, and Orphan Drug designations by the FDA. Additional information on the ANTLER trial (NCT04637763) can be found at clinicaltrials.gov.About Caribou’s novel next-generation CRISPR platform
CRISPR genome editing uses easily designed, modular biological tools to make DNA changes in living cells. There are two basic components of Class 2 CRISPR systems: the nuclease protein that cuts DNA and the RNA molecule(s) that guide the nuclease to generate a site-specific, double-stranded break, leading to an edit at the targeted genomic site. CRISPR systems are capable of editing unintended genomic sites, known as off-target editing, which may lead to harmful effects on cellular function and phenotype. In response to this challenge, Caribou has developed CRISPR hybrid RNA-DNA guides (chRDNAs; pronounced “chardonnays”) that direct substantially more precise genome editing compared to all-RNA guides. Caribou is deploying the power of its chRDNA technology to carry out high efficiency multiple edits, to develop CRISPR-edited therapies.About Caribou Biosciences, Inc.
Caribou Biosciences is a clinical-stage CRISPR genome-editing biopharmaceutical company dedicated to developing transformative therapies for patients with devastating diseases. The company’s genome-editing platform, including its Cas12a chRDNA technology, enables superior precision to develop cell therapies that are armored to potentially improve antitumor activity. Caribou is advancing a pipeline of clinical-stage off-the-shelf cell therapies from its CAR-T cell platform as readily available treatments for patients with hematologic malignancies and autoimmune diseases. Follow us @CaribouBio and visit www.cariboubio.com.Forward-looking statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential,” or “continue,” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. These forward-looking statements include, without limitation, statements related to Caribou’s strategy, plans, and objectives, and expectations regarding the timing of status and updates from its ANTLER Phase 1 clinical trial for CB-010, including expectations regarding the enrollment of 20 additional 2L LBCL patients to further study partial HLA matching outcomes, the timing of reporting of initial data from both 2L LBCL and CD 19 relapsed cohorts, the timing of reporting additional dose expansion data from the ANTLER trial, and the timing of initiation of a pivotal Phase 3 clinical trial for CB-010 in 2L LBCL patients, including the conditions to meet that timeline. Management believes that these forward-looking statements are reasonable as and when made. However, such forward-looking statements are subject to risks and uncertainties, and actual results may differ materially from any future results expressed or implied by the forward-looking statements. Risks and uncertainties include, without limitation, risks inherent in the development of cell therapy products; uncertainties related to the initiation, cost, timing, progress, and results of Caribou’s research and development programs, preclinical studies, and clinical trials; and the risk that initial, preliminary, or interim clinical trial data will not ultimately be predictive of the safety and efficacy of Caribou’s product candidates or that clinical outcomes may differ as patient enrollment continues and as more patient data becomes available and is fully evaluated; the ability to obtain key regulatory input and approvals as well as other risk factors described from time to time in Caribou’s filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2023 and subsequent filings. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. Except as required by law, Caribou undertakes no obligation to update publicly any forward-looking statements for any reason.Caution should be exercised when interpreting results from separate trials involving other CAR-T cell therapies. The results of other CAR-T cell therapies presented or referenced in this press release have been derived from publicly available reports of clinical trials not conducted by Caribou, and Caribou has not performed any head-to-head trials comparing any of these other CAR-T cell therapies with CB-010. As such, the results of these other clinical trials may not be comparable to clinical results for CB-010. The design of these other clinical trials varies in material ways from the design of the ANTLER clinical trial for CB-010, including with respect to patient populations, follow-up times, clinical trial phases, and subject characteristics. As a result, cross-trial comparisons may have no interpretive value on Caribou’s existing or future clinical results. For further information and to understand these material differences, you should read the reports for the other CAR-T cell therapy clinical trials and the sources included in the webcast slide presentation.
Caribou Biosciences, Inc. contacts:
Investors:
Amy Figueroa, CFA
investor.relations@cariboubio.comMedia:
Peggy Vorwald, PhD
media@cariboubio.com